At Nervana Medical in Sandy, Utah, we are committed to offering innovative, evidence-based therapies that extend beyond traditional weight loss. Glucagon-like peptide-1 (GLP-1) receptor agonists and glucose-dependent insulinotropic polypeptide (GIP) receptor agonists are best known for their role in managing type 2 diabetes and obesity, but emerging research suggests they may also influence chronic inflammation. With locations and telehealth services across Utah, Arizona, Idaho, Nevada, Montana, Wyoming, and California, our patients have access to treatments that may not only support metabolic health but also improve systemic and organ-specific inflammatory conditions.
Glucagon-like peptide-1 (GLP-1) receptor agonists and glucose-dependent insulinotropic polypeptide (GIP) receptor agonists do improve chronic inflammatory conditions, with evidence for both systemic and organ-specific anti-inflammatory effects.
GLP-1 receptor agonists have demonstrated significant reductions in circulating inflammatory biomarkers such as C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β) in patients with type 2 diabetes, as shown in a meta-analysis of 52 randomized controlled trials.[1] Mechanistically, GLP-1R activation modulates immune responses via central neuronal pathways and direct effects on intraepithelial lymphocytes, leading to suppression of peripheral inflammation.[2-3] These agents also show promise in reducing inflammation in rheumatic diseases, including rheumatoid arthritis and psoriatic arthritis, though clinical impact may be partially mediated by weight loss and metabolic improvements.[4-5]
GIP receptor agonists, and especially dual GIPR/GLP-1R agonists (e.g., tirzepatide), have demonstrated attenuation of inflammation in preclinical models of atherosclerosis and obesity-induced adipose tissue inflammation, with reductions in pro-inflammatory cytokines and immune cell infiltration.[6-7] GIPR signaling also alleviates gut inflammation in murine models, suggesting a direct immunomodulatory role.[8]
Combined GIPR/GLP-1R agonism appears to be superior to single agonism for both metabolic and anti-inflammatory effects, with translational relevance for cardiometabolic and inflammatory disease management.[6][8]
While these findings are robust in metabolic and preclinical inflammatory models, clinical data in non-metabolic chronic inflammatory diseases remain limited and further research is needed to establish efficacy and safety in broader population
The latest clinical trial data evaluating glucagon-like peptide-1 receptor agonists (GLP-1 RAs) and glucose-dependent insulinotropic polypeptide receptor agonists (GIP RAs) in non-metabolic chronic inflammatory diseases such as rheumatoid arthritis (RA) and inflammatory bowel disease (IBD show early signals of anti-inflammatory benefit, but robust, disease-modifying effects remain unproven and require further investigation.(4,5)
For rheumatoid arthritis and other rheumatic diseases, GLP-1 RAs have demonstrated reductions in inflammatory cytokines and some improvement in disease activity in small clinical studies and retrospective analyses, but most effects are confounded by weight loss and metabolic improvement. Disease-modifying effects are suggested, but head-to-head trials against standard immunomodulatory therapies are lacking, and long-term safety data are not yet available.[1-4] Mendelian randomization analyses suggest a protective effect of GLP-1R signaling on RA risk, but also signal possible increased risk for other autoimmune conditions such as ulcerative colitis and psoriasis.[4]
For inflammatory bowel disease, retrospective cohort studies and small clinical series indicate that GLP-1 RAs are generally safe, with a reduction in C-reactive protein and improved metabolic parameters, but no significant change in endoscopic scores or hospitalization rates has been demonstrated to date.[5-7] Preclinical data support anti-inflammatory and gut barrier-enhancing effects, but prospective randomized controlled trials are needed to establish efficacy and optimal patient selection.[5-6][8]
No large-scale randomized controlled trials have yet established GLP-1 or GIP receptor agonists as standard therapy for non-metabolic chronic inflammatory diseases. Their use remains investigational, with ongoing studies needed to clarify direct immunomodulatory effects, long-term safety, and comparative efficacy.[1-6][8-9]
While research is still evolving, GLP-1 and GIP therapies show promise as future tools in managing chronic inflammatory diseases such as rheumatoid arthritis, inflammatory bowel disease, and other autoimmune conditions. At Nervana Medical in Sandy, Utah, and through our telehealth programs across Arizona, Idaho, Nevada, Montana, Wyoming, and California, we take a personalized approach to care, using the latest science to guide safe and effective treatment options. If you are interested in exploring how GLP-1 or dual GLP-1/GIP therapy could support your health beyond weight loss, our team is here to help you take the next step.
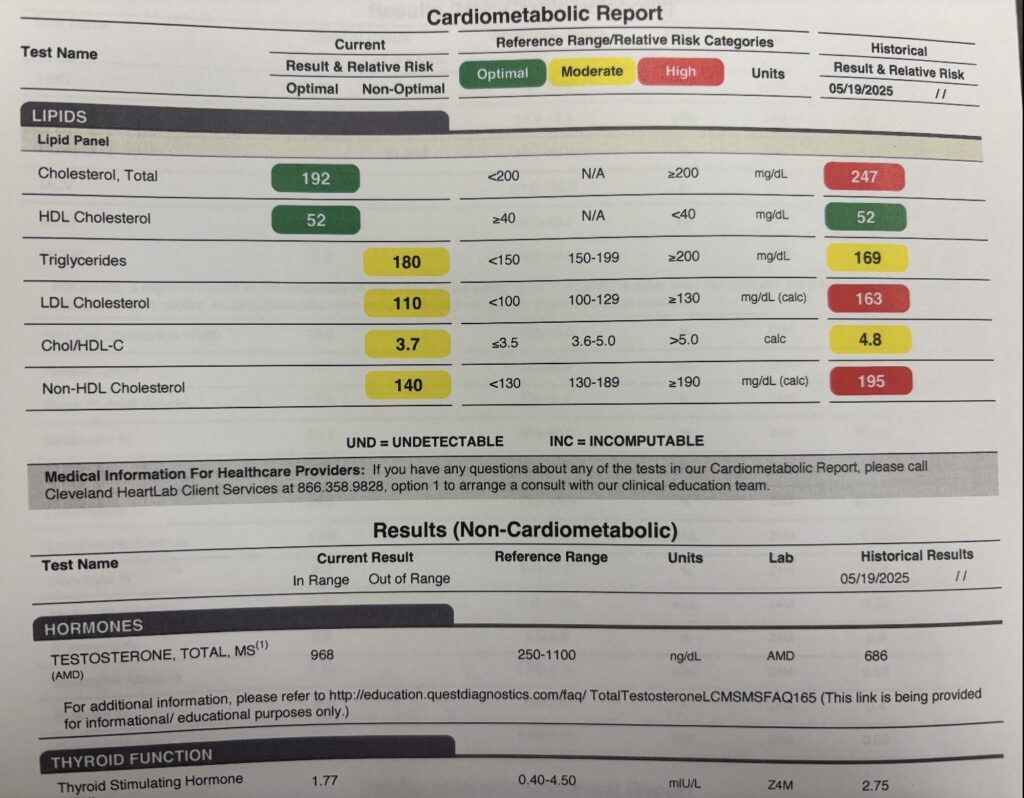
*Results from one of Nervana’s GLP1-GIP patients who is a pro cyclist and did not want to lose weight but has a PMH significant for RA on methotrexate and familial hyperlipidemia (healthy eater as noted by HDL)- Provider: Cassie D., AG-ACNP microdosed GLP1-GIP as he did not need to lose weight.
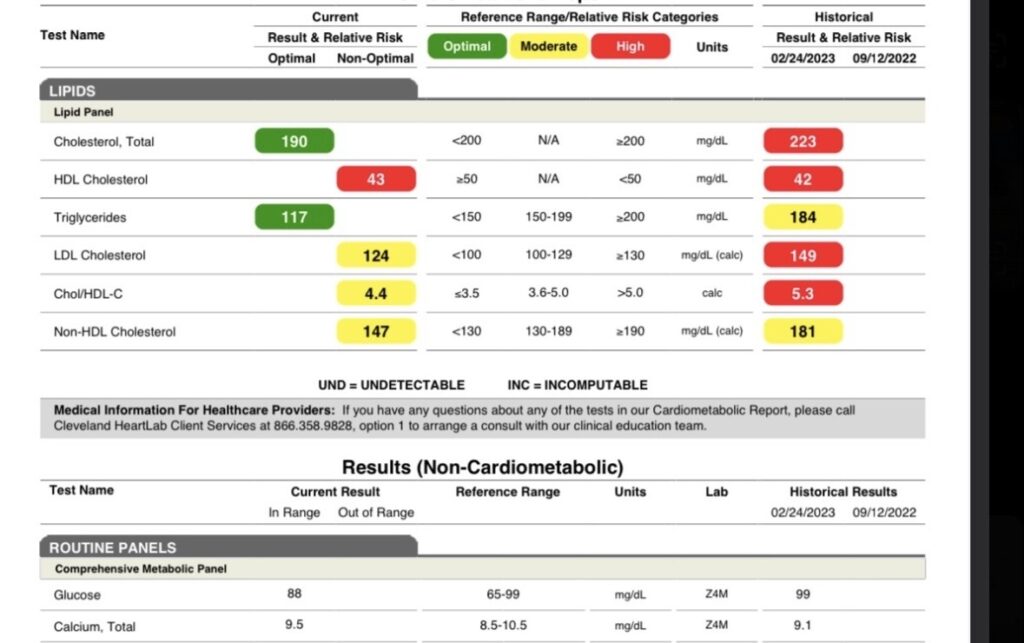
*Results from one of Nervana’s GLP1-GIP patients who did not want to lose weight but has a PMH significant for cardiovascular disease with adverse reactions to statins as well as a familial cardiovascular risk factor (father died of an MI)- Provider: Cassie D., AG-ACNP microdosed GLP1 as he did not need to lose weight. (Historical date of labs in 2023 as provider felt GLP1’s had an immunomodulatory effect prior to it being studied more).
References
Ren Y, Chen Y, Zheng W, et al.
Diabetes, Obesity & Metabolism. 2025;27(7):3607-3626. doi:10.1111/dom.16366.
New Research
Wong CK, McLean BA, Baggio LL, et al.
Cell Metabolism. 2024;36(1):130-143.e5. doi:10.1016/j.cmet.2023.11.009.
Leading Journal
3.Immunomodulation and Inflammation: Role of GLP-1R and GIPR Expressing Cells Within the Gut.
Morrow NM, Morissette A, Mulvihill EE.
Peptides. 2024;176:171200. doi:10.1016/j.peptides.2024.171200.
Bilgin E, Venerito V, Bogdanos DP.
Autoimmunity Reviews. 2025;24(9):103864. doi:10.1016/j.autrev.2025.103864.
Leading Journal New Research
5.What Clinicians Need to Know About Glucagon-Like Peptide 1 Agonists.
da Silva DLF, Singla S, Mease PJ.
The Journal of Rheumatology. 2025;:jrheum.2025-0531. doi:10.3899/jrheum.2025-0531.
New Research
van Eenige R, Ying Z, Tramper N, et al.
Atherosclerosis. 2023;372:19-31. doi:10.1016/j.atherosclerosis.2023.03.016.
Varol C, Zvibel I, Spektor L, et al.
Journal of Immunology (Baltimore, Md. : 1950). 2014;193(8):4002-9. doi:10.4049/jimmunol.1401149.
8.Glucose-Dependent Insulinotropic Polypeptide Receptor Signaling Alleviates Gut Inflammation in Mice.
Hammoud R, Kaur KD, Koehler JA, et al.
JCI Insight. 2024;10(3):e174825. doi:10.1172/jci.insight.174825.
Leading Journal New Research
Bilgin E, Venerito V, Bogdanos DP.
Autoimmunity Reviews. 2025;24(9):103864. doi:10.1016/j.autrev.2025.103864.
Leading Journal New Research
2.Antiobesity Medications in Rheumatology. Quo Vadis?.
Kyriazi N, Vassilakis KD, Bakiri A, Iliopoulos A, Fragoulis GE.
Annals of the Rheumatic Diseases. 2025;:S0003-4967(25)04312-2. doi:10.1016/j.ard.2025.08.013.
Leading Journal New Research
3.What Clinicians Need to Know About Glucagon-Like Peptide 1 Agonists.
da Silva DLF, Singla S, Mease PJ.
The Journal of Rheumatology. 2025;:jrheum.2025-0531. doi:10.3899/jrheum.2025-0531.
New Research
Yang Y, Liu W, Zhang Z, et al.
Journal of Autoimmunity. 2025;153:103414. doi:10.1016/j.jaut.2025.103414.
Leading Journal New Research
Colwill M, Povlsen S, Pollok R, et al.
Journal of Crohn’s & Colitis. 2025;:jjaf167. doi:10.1093/ecco-jcc/jjaf167.
New Research
6.GLP-1 Receptor Agonists in IBD: Exploring the Crossroads of Metabolism and Inflammation.
Migliorisi G, Gabbiadini R, Dal Buono A, et al.
Frontiers in Immunology. 2025;16:1610368. doi:10.3389/fimmu.2025.1610368.
Leading Journal New Research
7.Safety and Effectiveness of Glucagon-Like Peptide-1 Receptor Agonists in Inflammatory Bowel Disease.
Anderson SR, Ayoub M, Coats S, et al.
The American Journal of Gastroenterology. 2025;120(5):1152-1155. doi:10.14309/ajg.0000000000003208.
New Research
8.Immunomodulation and Inflammation: Role of GLP-1R and GIPR Expressing Cells Within the Gut.
Morrow NM, Morissette A, Mulvihill EE.
Peptides. 2024;176:171200. doi:10.1016/j.peptides.2024.171200.
9.New Molecules and Indications for GLP-1 Medicines.
Gonzalez-Rellan MJ, Drucker DJ.
JAMA. 2025;:2838996. doi:10.1001/jama.2025.14392.
Leading Journal New Research

