Testosterone therapy has become a cornerstone of hormone optimization for many men and women, improving energy, strength, body composition, and overall quality of life. However, one of the most common and challenging side effects of testosterone replacement therapy (TRT) is secondary polycythemia, an increase in red blood cells circulating the body.
While polycythemia (too many) sounds like the opposite of anemia (too few), many patients are surprised to learn that they can develop iron deficiency anemia even in the presence of elevated hematocrit and hemoglobin levels. This paradox highlights the importance of careful monitoring and individualized management for patients undergoing TRT.
At Nervana Medical in Sandy, Utah, we specialize in advanced hormone optimization and integrative care, helping patients achieve the benefits of testosterone therapy while avoiding common complications such as secondary polycythemia and iron deficiency. Many men are surprised to learn that they can experience iron deficiency anemia even when their hematocrit is elevated from testosterone replacement therapy or chronic hypoxia (such as untreated sleep apnea). This paradox occurs because while testosterone and low oxygen states stimulate the body to make more red blood cells, the increased demand for iron often outpaces supply; especially when therapeutic phlebotomy is used. Understanding this unique balance is essential for safe, effective hormone management, and our team at Nervana Medical provides personalized strategies including careful monitoring, dosing adjustments, and iron repletion to optimize health and performance.
Why Testosterone Therapy Causes Polycythemia
Testosterone stimulates erythropoiesis (red blood cell production), partly through increasing erythropoietin and enhancing bone marrow activity. Elevated red blood cell counts may improve oxygen delivery, but when hematocrit rises too high, blood viscosity increases, raising the risk of headaches, fatigue, clotting events, and cardiovascular complication (El-Khatib et al., 2022; Bhasin & Snyder, 2025).
To reduce hematocrit, therapeutic phlebotomy (blood donation or removal) is often recommended. However, each 500 mL phlebotomy removes approximately 200–250 mg of iron (Kiss et al., 2015). Frequent phlebotomies can deplete iron stores, leading to iron deficiency anemia despite the persistence of polycythemia.
The Iron Deficiency Paradox in Secondary Polycythemia
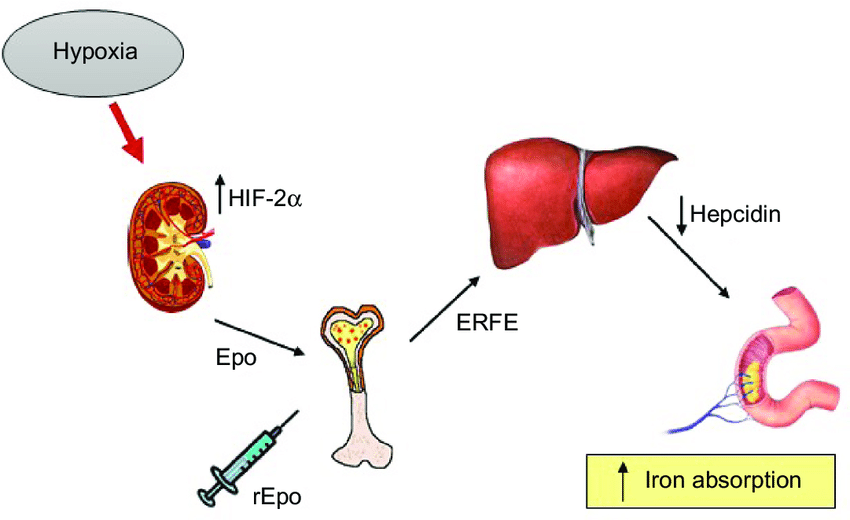
Conditions causing secondary polycythemia such as testosterone therapy and chronic hypoxia (from cardiac or lung disease or even from issues such as untreated obstructive sleep apnea) drive persistent erythropoietin release.Mechanistically, increased erythropoietic activity in response to hypoxia suppresses hepcidin, increasing iron absorption, but if iron supply cannot meet demand, iron deficiency anemia develops despite polycythemia. Figure 1 from the New England Journal of Medicine illustrates the adaptive mechanisms of iron metabolism in response to anemia and increased erythropoiesis, highlighting how iron deficiency can persist even with increased red cell production.
When iron stores cannot meet the increased demand, patients develop microcytic, hypochromic red cells, meaning that even though they have “too many” red blood cells, those cells are poorly hemoglobinized and less effective at oxygen delivery (Bessman, 1977; Camaschella, 2015). To assess microcytic hypochromic red blood cells, you would look at your serum red cell indices; the key parameters are:
1. MCV (Mean Corpuscular Volume)
- Measures the average size of red blood cells.
- Microcytic = low MCV (<80 fL in adults).
Suggests iron deficiency anemia, thalassemia, or anemia of chronic disease.
2. MCH (Mean Corpuscular Hemoglobin)
- Reflects the average amount of hemoglobin per red blood cell.
Low MCH = hypochromia (cells carry less hemoglobin).
3. MCHC (Mean Corpuscular Hemoglobin Concentration)
- Measures the hemoglobin concentration within each red cell.
- Low MCHC = hypochromic RBCs (cells look pale on smear).
4. RDW (Red Cell Distribution Width)
- Shows the variation in RBC size.
- High RDW is common in iron deficiency anemia (mixed small + normal cells).
- RDW is often normal in thalassemia trait despite microcytosis.
5. Peripheral Blood Smear (often ordered if indices are abnormal)
- Confirms findings: small, pale cells in microcytic, hypochromic anemia.
- May also show anisocytosis (different sizes) and poikilocytosis (different shapes).
The American Heart Association notes that iron deficiency is common in secondary erythrocytosis and should be identified and corrected, as untreated deficiency worsens fatigue, reduces exercise tolerance, and can paradoxically impair oxygen delivery (Lui et al., 2017; Stout et al., 2019).
Clinical studies and ferritin kinetics show that after a 500 mL donation, serum ferritin decreases by about 30 ng/mL over 30 days, and each ng/mL of ferritin corresponds to 8–10 mg of storage iron. Thus, the total iron loss per donation is calculated as 30 ng/mL × 8 mg = 240 mg, which aligns with direct measurements of red cell iron content and is widely cited in transfusion medicine literature.This iron loss can take several months to fully replace through diet alone, and repeated donations without supplementation significantly increase the risk of iron deficiency, especially in women and frequent donors.
The following figure illustrates the drop in ferritin (and thus iron stores) after blood donation and quantifies the iron loss per unit:
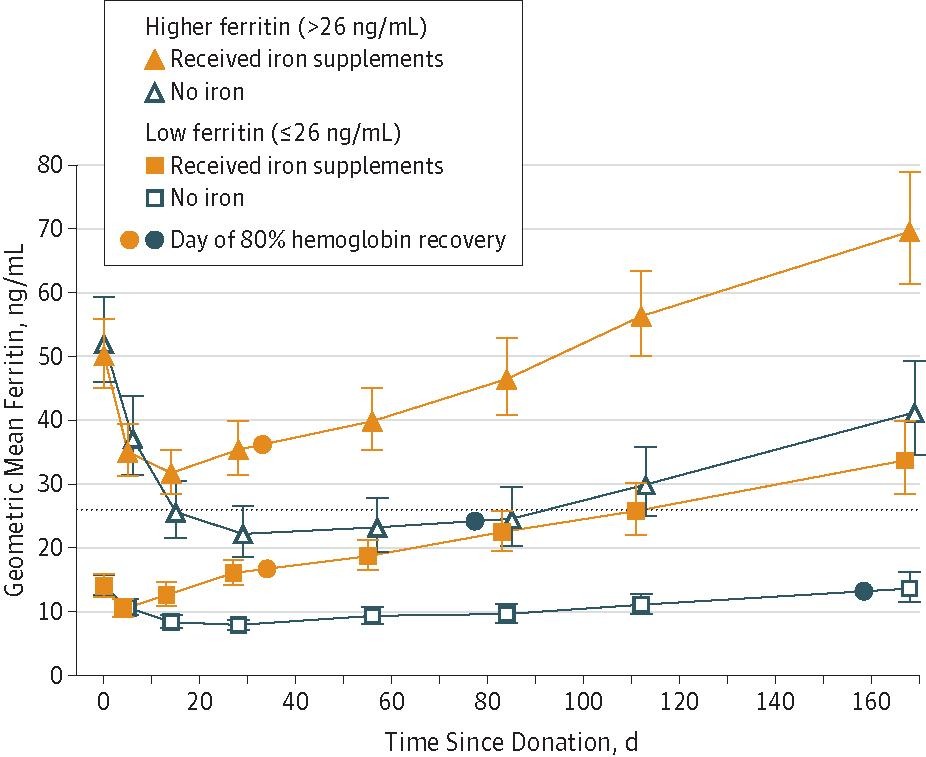
At Nervana Medical, we often see this pattern in men on testosterone therapy who undergo repeated phlebotomy and need iron treatment to battle their fatigue despite having optimized their testosterone. In these cases, iron deficiency can develop even when hematocrit remains elevated.
The Role of Testosterone Dosing Frequency
One strategy to reduce the risk of polycythemia is adjusting how testosterone is administered. Switching from once-weekly to twice-weekly injections smooths out testosterone peaks and troughs, resulting in less stimulation of red blood cell production (El-Khatib et al., 2022; Okano et al., 2025).
Switching from once-weekly to twice-weekly testosterone injections (splitting the total dose) can modestly reduce peak serum testosterone levels and may lower the risk of secondary polycythemia, as higher peaks are associated with greater hematocrit increases(El-Khatib et al., 2022; Bhasin & Snyder, 2025; Nackeeran et al., 2022). This approach aims to smooth out fluctuations in testosterone concentrations, which are linked to erythrocytosis.
Hematocrit typically begins to decrease within weeks after dose adjustment, but most studies report significant changes in hematocrit over 3–6 months of monitoring following regimen changes(Okano et al., 2025; Bhasin et al., 2018). The reduction in hematocrit after dose fractionation is generally modest; for example, a randomized trial found that splitting the dose resulted in a decrease of less than 1% in hematocrit over 3 months, while more substantial reductions required further dose lowering or temporary suspension of therapy(Okano et al., 2025).
Dose fractionation preserves therapeutic testosterone levels while offering only modest reductions in hematocrit; if a greater decrease is needed, additional interventions may be required(Okano et al., 2025). The Endocrine Society recommends close monitoring of hematocrit at baseline, 3–6 months after any dosing change, and annually thereafter to assess response and guide further management (Bhasin et al., 2018)..
There is limited direct evidence on the exact time course of hematocrit decline after switching to twice-weekly dosing, but most clinical changes are observed within the first 3 months. Continued monitoring is essential to ensure hematocrit remains within safe limits and to adjust therapy as needed.
Iron Replacement in the Setting of Polycythemia
It may seem counterintuitive to give iron to someone with elevated hematocrit, but studies confirm that iron repletion is safe and necessary in patients with secondary polycythemia who are iron deficient (Lui et al., 2017; Auerbach et al., 2025).
- Oral iron (150–200 mg elemental daily or alternate-day dosing) is usually first-line.
- IV iron is considered for patients who cannot tolerate oral formulations or who need rapid correction.
- Monitoring includes ferritin, transferrin saturation, and hematocrit every few months.
- We recommend a patient to fast for iron studies as inflammation can cause ferritin levels to be elevated, so also do not have them drawn during active infection.
At Nervana Medical, we combine hormone optimization with nutrient repletion, including IV iron and personalized supplementation protocols, to ensure patients achieve not only the benefits of testosterone but also the optimal oxygen-carrying capacity of their blood.
Key Takeaways for Patients
- Yes, you can be iron deficient even with polycythemia.
- Therapeutic phlebotomy lowers hematocrit but also depletes iron stores.
- Twice-weekly dosing of testosterone can help smooth hematocrit levels but may not fully prevent erythrocytosis.
- Untreated hypoxia (e.g., from non-compliance with CPAP for sleep apnea) can exacerbate secondary polycythemia.
- Iron replacement is safe and improves symptoms when deficiency is identified.
Nervana Medical’s Approach
At Nervana Medical, we understand the complex interplay between testosterone therapy, polycythemia, and iron deficiency. Through individualized treatment plans that may include dose fractionation, weight loss to potentially improve sleep apnea, iron monitoring, and replacement therapy, our patients experience the benefits of testosterone while minimizing risks. If you’re receiving TRT and struggling with fluctuating hematocrit, fatigue, or iron-related symptoms, our expert providers can help you restore balance and achieve optimal wellness. Contact Nervana Medical today to learn how our comprehensive approach to hormone health, iron support, and metabolic optimization can help you feel and function at your best. We offer hormone only memberships as well as a medical concierge style multi-modality, multi disciplinary comprehensive approach for helping you live your best life!
References
- Bessman JD. Microcytic Polycythemia. JAMA. 1977;238(22):2391-2392. doi:10.1001/jama.238.22.2391.
- Giglia TM, Massicotte MP, Tweddell JS, et al. Prevention and Treatment of Thrombosis in Pediatric and Congenital Heart Disease. Circulation. 2013;128(24):2622-703.
- Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC Guideline for Adults With Congenital Heart Disease. J Am Coll Cardiol. 2019;73(12):e81-e192.
- Lui GK, Saidi A, Bhatt AB, et al. Noncardiac Complications in Adults With Congenital Heart Disease. Circulation. 2017;136(20):e348-e392.
- Camaschella C. Iron-Deficiency Anemia. N Engl J Med. 2015;372:1832-43.
- El-Khatib FM, Huynh LM, Kopelevich A, et al. Comparative Assessment of Testosterone Regimens. Int J Impot Res. 2022;34(6):558-563.
- Bhasin S, Snyder PJ. Testosterone Treatment in Hypogonadism. N Engl J Med. 2025;393(6):581-591.
- Okano SHP, Franceschini SA, Cantelli DAL, et al. Effect of Testosterone Cypionate in Erythrocytosis. J Sex Med. 2025.
- Kiss JE, Brambilla D, Glynn SA, et al. Oral Iron Supplementation After Blood Donation: A Randomized Clinical Trial. JAMA. 2015;313(6):575-83.
Auerbach M, DeLoughery TG, Tirnauer JS. Iron Deficiency in Adults. JAMA. 2025;333(20):1813-1823.

